Abstract
Background: An ambitious initiative underway in the inherited bleeding disorder (IBD) community aims to create a national research blueprint that can help accelerate research progress and address important gaps in care, particularly within rare disorders and underserved populations. Led by the National Hemophilia Foundation (NHF), the effort is defined by input from across the community, including research leaders, patient subject matter experts (SMEs), caregivers, allied health professionals and specialists, and industry. Two foundational principles of the blueprint are that a) it must deliver on key issues that most significantly impact the lives of those affected by an IBD, and b) the priorities defined are relevant and actionable in order to provoke real and lasting changes in the care paradigm.
Methods: To ensure the blueprint accurately reflects the most pressing needs from across the community, NHF has enlisted the support of diverse segments of the population throughout the process.
Listening: NHF coordinated a comprehensive, community-wide listening exercise, including focus groups, virtual listening sessions, and consumer and professional surveys, to collect insights that have shaped and guided the blueprint development.
Engagement: Representatives from across the IBD community have been enlisted to participate in the development process through enrollment in one of six interdisciplinary working groups (WGs), each focusing on broad themes raised during the listening exercises. (Table 1) In total, 164 individuals are participating in the WGs, including chapter representatives, allied healthcare providers, researchers, federal partners and other IBD organizations. Each WG also features experts outside the IBD community who can introduce innovations from other fields. Finally, each WG includes the participation of subject matter experts (SMEs), individuals affected by bleeding disorders who provide personal perspectives on the value and potential impact of the proposed research priorities. NHF is actively supporting these groups with regular engagement, guidance, and recommendations while encouraging robust dialogue to distill critical priority research areas.
To ensure the blueprint is well defined and actionable, NHF has devised a rigorous development and refinement process.
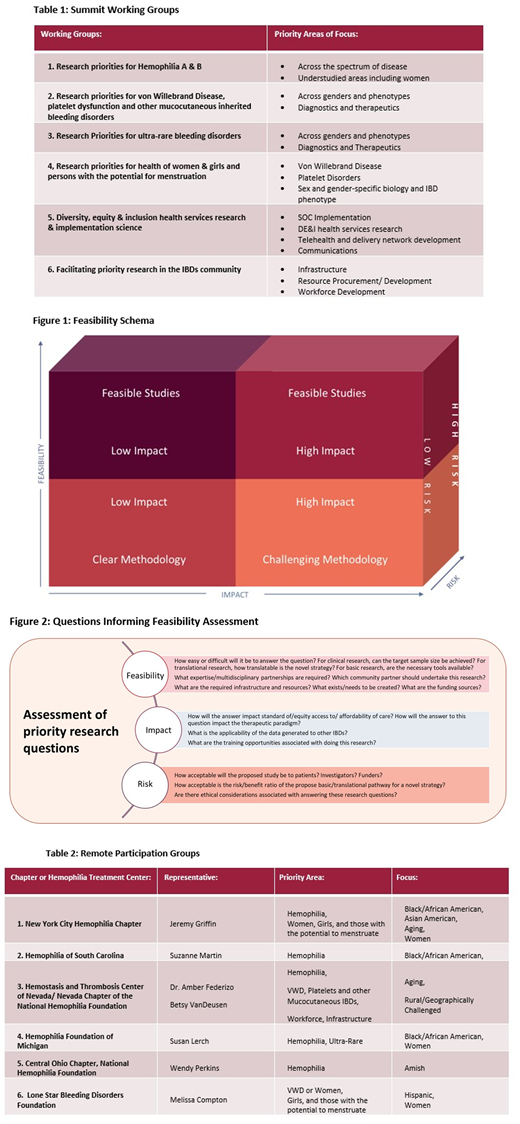
Feasibility Assessment: Together with expert advisers, NHF has defined a set of feasibility criteria to help the WGs address potential opportunities based on three key areas: (see Figures 1 and 2)
Feasibility assesses the difficulty in answering the proposed question, including required expertise, infrastructure, and resources.
Impact estimates the change we can foster through the priority.
And risk considers the challenges of the research question, such as the risk/benefit ratio for novel strategies and any ethical considerations.
Each research priority or model is scored based on these areas, and the combined evaluation will determine how they are included and prioritized in the blueprint.
Summit: Upon completion of the WG assessments, NHF will bring the community together for a State of the Science (SOS) Research Summit, September 12-15, 2021, during which each WG will summarize their recommendations for live, interactive discussion. During each session, panels will discuss the recommendations and collect feedback from community participants, as well as from remote participation groups comprised of representatives from underserved segments of the population.
Results: The discussions from the working groups and Research Summit will be consolidated into a series of manuscripts and published as a community-driven national research blueprint in mid-2022. The voices of individuals affected by IBDs have been the central driver in this process, from the listening activities and WGs to the planned SOS, and the community will continue to champion the efforts defined in the blueprint.
Conclusions: This initiative represents an opportunity to catalyze impactful change in the treatment of IBDs. To ensure its success, NHF has methodically enlisted broad community involvement and driven a rigorous prioritization process in order to identify specific and actionable topics that will help guide research plans for the IBD community. Our hope is that this blueprint will help shepherd advances in care that could fundamentally redefine the experience of living with these disorders.
Witkop: Teralmmune, Inc.: Consultancy. Recht: Sanofi: Consultancy; Octapharma: Consultancy; Novo Nordisk: Consultancy; Foundation for Women and Girls with Blood Disorders, Partners in Bleeding Disorders: Speakers Bureau; American Thrombosis and Hemostasis Network: Current Employment; Oregon Health & Science University: Current Employment; Genentech: Consultancy; Hema Biologics: Consultancy; CSL Behring: Consultancy; Takeda: Consultancy; uniQure: Consultancy; Catalyst Biosciences: Consultancy; Pfizer: Consultancy; Kedrion: Consultancy. Valentino: Spark: Ended employment in the past 24 months.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal